(구)글로벌 핫이슈
방사선종양학
- [J Clin Oncol.] Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma.
Memorial Sloan Kettering Cancer Center / Andreas Rimner*
- 출처
- J Clin Oncol.
- 등재일
- 2016 Aug 10
- 저널이슈번호
- 34(23):2761-8. doi: 10.1200/JCO.2016.67.2675. Epub 2016 Jun 20.
- 내용
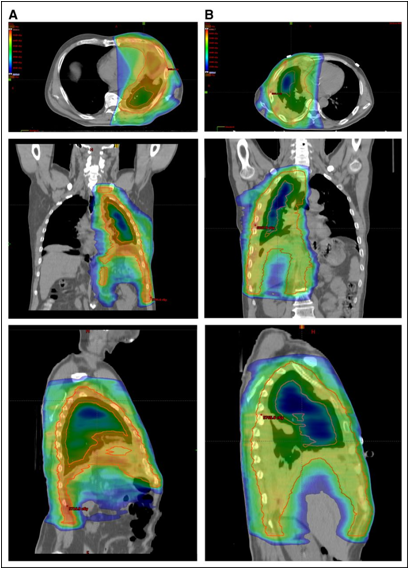
Example of hemithoracic pleural intensity-modulated radiation therapy (IMRT) in a patient with (A) a resected and (B) an unresectable tumor.

Acute and Late IMRT Toxicity
Abstract
PURPOSE:
We conducted a two-center phase II study to determine the safety of hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) after chemotherapy and pleurectomy-decortication (P/D) as part of a multimodality lung-sparing treatment.
PATIENTS AND METHODS:
Patients received up to four cycles of pemetrexed plus platinum. If feasible, P/D was performed. Hemithoracic IMPRINT was administered to a planned dose of 50.4 Gy in 28 fractions. The primary end point was the incidence of grade 3 or greater radiation pneumonitis (RP).
RESULTS:
A total of 45 patients were enrolled; 18 were not evaluable (because of disease progression before radiation therapy [RT], n = 9; refusal of surgery or RT, n = 5; extrapleural pneumonectomy at time of surgery, n = 2; or chemotherapy complications, n = 2). A total of 26 patients received pemetrexed plus cisplatin, 18 received pemetrexed plus carboplatin, and four received a combination. Thirteen patients (28.9%) had a partial response, 15 patients (33.3%) experienced disease progression, one patient died during chemotherapy, and all others had stable disease. Eight patients underwent P/D or an extended P/D, and 13 underwent a partial P/D. A total of 27 patients started IMPRINT (median dose, 46.8 Gy; range, 28.8 to 50.4 Gy) and were evaluable for the primary end point (median follow-up, 21.6 months). Six patients experienced grade 2 RP, and two patients experienced grade 3 RP; all recovered after corticosteroid initiation. No grade 4 or 5 radiation-related toxicities were observed. The median progression-free survival and overall survival (OS) were 12.4 and 23.7 months, respectively; the 2-year OS was 59% in patients with resectable tumors and was 25% in patients with unresectable tumors.
CONCLUSIONS:
Hemithoracic IMPRINT for malignant pleural mesothelioma (MPM) is safe and has an acceptable rate of RP. Its incorporation with chemotherapy and P/D forms a new lung-sparing treatment paradigm for patients with locally advanced MPM.
Author information
Rimner A1, Zauderer MG2, Gomez DR2, Adusumilli PS2, Parhar PK2, Wu AJ2, Woo KM2, Shen R2, Ginsberg MS2, Yorke ED2, Rice DC2, Tsao AS2, Rosenzweig KE2, Rusch VW2, Krug LM2.
1Andreas Rimner, Marjorie G. Zauderer, Prasad S. Adusumilli, Preeti K. Parhar, Abraham J. Wu, Kaitlin M. Woo, Ronglai Shen, Michelle S. Ginsberg, Ellen D. Yorke, Kenneth E. Rosenzweig, Valerie W. Rusch, and Lee M. Krug, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical Center, New York, NY; and Daniel R. Gomez, David C. Rice, and Anne S. Tsao, MD Anderson Cancer Center, Houston, TX rimnera@mskcc.org.
2Andreas Rimner, Marjorie G. Zauderer, Prasad S. Adusumilli, Preeti K. Parhar, Abraham J. Wu, Kaitlin M. Woo, Ronglai Shen, Michelle S. Ginsberg, Ellen D. Yorke, Kenneth E. Rosenzweig, Valerie W. Rusch, and Lee M. Krug, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical Center, New York, NY; and Daniel R. Gomez, David C. Rice, and Anne S. Tsao, MD Anderson Cancer Center, Houston, TX.
- 덧글달기
- 이전글 [J Clin Oncol.] Improved Survival With Prostate Radiation in Addition to Androgen Deprivation Therapy for Men With Newly Diagnosed Metastatic Prostate Cancer.
- 다음글 [Lancet Oncol.] Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial.









