글로벌 연구동향
방사선생물학
![[Oncotarget.] Overcoming HSP27-mediated resistance by altered dimerization of HSP27 using small molecules.](/enewspaper/upimages/admin_20160810105722_R.jpg) 2016년 08월호
2016년 08월호
[Oncotarget.] Overcoming HSP27-mediated resistance by altered dimerization of HSP27 using small molecules.이화여대, 차의과대/ 김지혜, 이윤실*, 나영화*
- 출처
- Oncotarget.
- 등재일
- 2016 Jul 16
- 저널이슈번호
- doi: 10.18632/oncotarget.10629.
- 내용
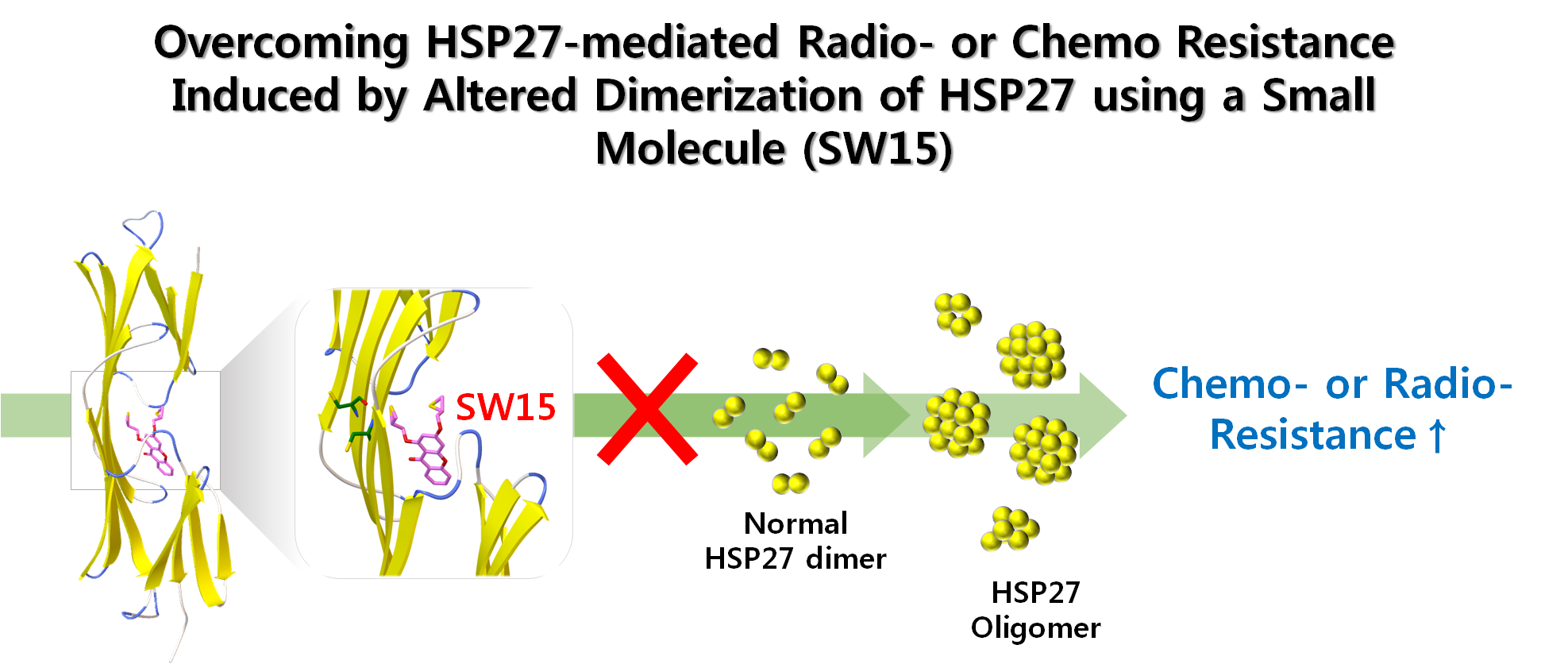
AbstractHeat shock protein 27 (HSP27, HSPB1) is an anti-apoptotic protein characterized for its tumorigenic and metastatic properties, and now referenced as a major therapeutic target in many types of cancer. The biochemical properties of HSP27 rely on a structural oligomeric and dynamic organization that is important for its chaperone activity. Down-regulation by small interfering RNA or inhibition with a dominant-negative mutant efficiently counteracts the anti-apoptotic and protective properties of HSP27. However, unlike other HSPs such as HSP90 and HSP70, small molecule approaches for neutralization of HSP27 are not well established because of the absence of an ATP binding domain. Previously, we found that a small molecule, zerumbone (ZER), induced altered dimerization of HSP27 by cross linking the cysteine residues required to build a large oligomer, led to sensitization in combination with radiation. In this study, we identified another small molecule, a xanthone compound, more capable of altering dimeric HSP27 than ZER and yielding sensitization in human lung cancer cells when combined with HSP90 inhibitors or standard anticancer modalities such as irradiation and cytotoxic anticancer drugs. Therefore, altered dimerization of HSP27 represents a good strategy for anticancer therapy in HSP27-overexpressing cancer cells.
Author information
Kim JH1, Jung YJ1, Choi B1, Lee NL1, Lee HJ2, Kwak SY3, Kwon Y1, Na Y3, Lee YS1.
1Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul, 120-720, Korea.
2Division of Radiation Effects, Korea Institute of Radiological and Medical Sciences, Seoul, 139-706, Korea.
3College of Pharmacy, CHA University, Pocheon, 487-010, Korea.
- 키워드
- HSP27 inhibition; altered dimerization; combination therapy; overcoming resistance
- 연구소개
- 열충격단백질 27 (HSP27)은 방사선 및 항암내성을 유도하는 대표적 단백질로 알려져 있으나 다른 HSP90 나 HSP70과는 달리 ATP binding domain이 존재하지 않아 초고속저해제 발굴이 불가능 하였음. 본 연구에서는 HSP27의 비이상적 이성체를 형성하는 저분자물질을 발굴하는 새로운 시스템을 이용하여 방사선 및 항암제에 의한 내성을 극복하는 HSP27 저해 저분자물질을 발굴한 논문으로써 HSP27에 의해 유도되는 다양한 내성을 극복할 수 있는 First in Class 약물개발에 대한 가능성 제시.
- 덧글달기
- 이전글 [Cancer Res Treat.] Underexpression of HOXA11 is Associated with Treatment Resistance and Poor Prognosis in Glioblastoma.
- 다음글 [Int J Radiat Oncol Biol Phys.] Real-time Tumor Oxygenation Changes After Single High-dose Radiation Therapy in Orthotopic and Subcutaneous Lung Cancer in Mice: Clinical Implication for Stereotactic Ablative Radiation Therapy Schedule Optimization.







