(구)글로벌 핫이슈
방사선종양학
- 2017년 12월호
[N Engl J Med.] Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer.H. Lee Moffitt Cancer Center and Research Institute / S.J. Antonia, A. Villegas*
- 출처
- N Engl J Med.
- 등재일
- 2017 Nov 16
- 저널이슈번호
- 377(20):1919-1929. doi: 10.1056/NEJMoa1709937. Epub 2017 Sep 8.
- 내용
Abstract
BACKGROUND:
Most patients with locally advanced, unresectable, non-small-cell lung cancer (NSCLC) have disease progression despite definitive chemoradiotherapy (chemotherapy plus concurrent radiation therapy). This phase 3 study compared the anti-programmed death ligand 1 antibody durvalumab as consolidation therapy with placebo in patients with stage III NSCLC who did not have disease progression after two or more cycles of platinum-based chemoradiotherapy.METHODS:
We randomly assigned patients, in a 2:1 ratio, to receive durvalumab (at a dose of 10 mg per kilogram of body weight intravenously) or placebo every 2 weeks for up to 12 months. The study drug was administered 1 to 42 days after the patients had received chemoradiotherapy. The coprimary end points were progression-free survival (as assessed by means of blinded independent central review) and overall survival (unplanned for the interim analysis). Secondary end points included 12-month and 18-month progression-free survival rates, the objective response rate, the duration of response, the time to death or distant metastasis, and safety.RESULTS:
Of 713 patients who underwent randomization, 709 received consolidation therapy (473 received durvalumab and 236 received placebo). The median progression-free survival from randomization was 16.8 months (95% confidence interval [CI], 13.0 to 18.1) with durvalumab versus 5.6 months (95% CI, 4.6 to 7.8) with placebo (stratified hazard ratio for disease progression or death, 0.52; 95% CI, 0.42 to 0.65; P<0.001); the 12-month progression-free survival rate was 55.9% versus 35.3%, and the 18-month progression-free survival rate was 44.2% versus 27.0%. The response rate was higher with durvalumab than with placebo (28.4% vs. 16.0%; P<0.001), and the median duration of response was longer (72.8% vs. 46.8% of the patients had an ongoing response at 18 months). The median time to death or distant metastasis was longer with durvalumab than with placebo (23.2 months vs. 14.6 months; P<0.001). Grade 3 or 4 adverse events occurred in 29.9% of the patients who received durvalumab and 26.1% of those who received placebo; the most common adverse event of grade 3 or 4 was pneumonia (4.4% and 3.8%, respectively). A total of 15.4% of patients in the durvalumab group and 9.8% of those in the placebo group discontinued the study drug because of adverse events.CONCLUSIONS:
Progression-free survival was significantly longer with durvalumab than with placebo. The secondary end points also favored durvalumab, and safety was similar between the groups. (Funded by AstraZeneca; PACIFIC ClinicalTrials.gov number, NCT02125461 .).Comment in
Immunotherapy for Unresectable Stage III Non-Small-Cell Lung Cancer. [N Engl J Med. 2017]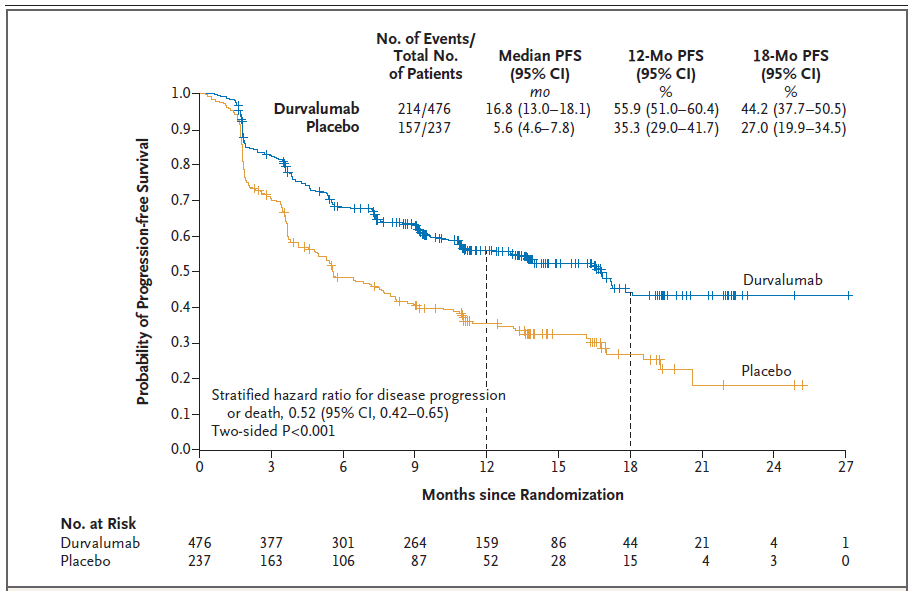
Figure 1. Progression-free Survival in the Intention-to-Treat Population.
Shown are Kaplan–Meier curves for progression-free survival (PFS), defined according to the Response Evaluation Criteria in Solid Tumors, version 1.1, and assessed by means of blinded independent central review. Tick marks indicate censored observations, and vertical lines indicate the times of landmark PFS analyses. The intention-to-treat population included all patients who underwent randomization.Author information
Antonia SJ1, Villegas A1, Daniel D1, Vicente D1, Murakami S1, Hui R1, Yokoi T1, Chiappori A1, Lee KH1, de Wit M1, Cho BC1, Bourhaba M1, Quantin X1, Tokito T1, Mekhail T1, Planchard D1, Kim YC1, Karapetis CS1, Hiret S1, Ostoros G1, Kubota K1, Gray JE1, Paz-Ares L1, de Castro Carpeño J1, Wadsworth C1, Melillo G1, Jiang H1, Huang Y1, Dennis PA1, Özgüroğlu M1; PACIFIC Investigators.
1From the H. Lee Moffitt Cancer Center and Research Institute, Tampa (S.J.A., A.C., J.E.G.), Cancer Specialists of North Florida, Jacksonville (A.V.), and Florida Hospital Cancer Institute, Orlando (T.M.) - all in Florida; Tennessee Oncology, Chattanooga, and Sarah Cannon Research Institute, Nashville - both in Tennessee (D.D.); Hospital Universitario Virgen Macarena, Seville (D.V.), and Hospital Universitario 12 de Octubre, Centro de Investigación Biomédica en Red de Cáncer, Universidad Complutense and the Spanish National Cancer Research Center (L.P.-A.), and Hospital Universitario La Paz (J.C.C.), Madrid - all in Spain; Kanagawa Cancer Center, Yokohama (S.M.), Kansai Medical University Hospital, Hirakata (T.Y.), Kurume University Hospital, Kurume (T.T.), and Nippon Medical School Hospital, Tokyo (K.K.) - all in Japan; Westmead Hospital and the University of Sydney, Sydney (R.H.), and Flinders University and Flinders Medical Centre, Bedford Park, SA (C.S.K.) - all in Australia; Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju (K.H.L.), Yonsei Cancer Center, Yonsei University College of Medicine, Seoul (B.C.C.), and Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Gwangju (Y.-C.K.) - all in South Korea; Vivantes Klinikum Neukölln, Berlin (M.W.); Centre Hospitalier Universitaire de Liège, Liège, Belgium (M.B.); Centre Hospitalier Universitaire Montpellier and Cancer Institute of Montpellier Val d'Aurelle, Montpellier (X.Q.), Institut Gustave Roussy, Villejuif (D.P.), and Institut de Cancérologie de l'Ouest-Site René Gauducheau, Saint Herblain (S.H.) - all in France; National Koranyi Institute of Pulmonology, Budapest, Hungary (G.O.); AstraZeneca, Alderley Park, United Kingdom (C.W.); AstraZeneca, Gaithersburg, MD (G.M., H.J., Y.H., P.A.D.); and Istanbul University Cerrahpasa School of Medicine, Istanbul, Turkey (M.Ö.).
Collaborators (225)
Jasas K, Obyrne K, Houghton B, Hughes B, Lewis C, Links M, Ng S, Parente P, Gauden S, Forget F, Vercauter P, Vansteenkiste J, Canon JL, Cheema P, Vincent M, Murray N, Rothenstein J, Zibdawi L, Bradbury P, Butts C, El-Maraghi R, Bebb D, Acevedo Gaete A, Orellana Ulunque EA, Aren Frontera OR, Mazières J, Renault PA, Robinet G, Cortot A, Hilgers W, Poudenx M, Barlesi F, El Kouri C, Perol M, Lena H, Sabatini M, Pujol JL, Laack E, Schulz C, Brugger W, Faehling M, Reck M, Wolff T, Fischer J, Emde TO, Scholz C, Rueckert A, Kalofonos H, Kotsakis A, Syrigos K, Papazisis K, Zarogoulidis K, Sztancsik Z, Losonczy G, Csanky E, Nechushtan H, Wollner M, Chella A, Bearz A, Chiuri VE, Garassino M, Gianni L, Brighenti M, Ciardiello F, Milella M, Soto Parra H, Sugawara S, Atagi S, Hirashima T, Imamura F, Iwamoto Y, Kanda S, Masuda N, Minato K, Nakagawa K, Niho S, Saka H, Takahashi T, Fujisaka Y, Sakai H, Takahashi K, Baba T, Harada M, Kasahara K, Maeda T, Maemondo M, Okamoto I, Takeda Y, Kobayashi K, Nogami N, Kiura K, Kato T, Hotta K, Park K, Cho EK, Kim HK, Lee JS, Son C, Kim SW, Shin SW, Kim JH, De Langen J, van den Borne B, Kloover JS, van den Heuvel M, van der Leest KH, Aerts JGJV, Hashemi S, Rodriguez Pantigoso W, Bermudez Alfaro V, Jassem J, Mandziuk S, Kowalski D, Chopra A, Tan EH, Chin TM, Soo RA, Stresko M, Demo P, Packan T, Chovanec J, Cohen G, Jacobs C, Rens D, Corral Jaime J, Vidal OJ, Taus Garcia A, Cardenal Alemany F, Garcia Gomez R, Holgado Martin E, Ponce Aix S, Porta Balanya R, Isla Casado D, Majem Tarruella M, Marquez Medina D, Alfaro Lizaso J, Blasco Cordellat A, Sanchez Torres JM, Massuti Sureda B, Sanchez A, Belda Iniesta C, Alvarez R, Hsia TC, Chen YM, Lee KY, Lien YC, Yang CH, Chung CL, Reungwetwattana T, Chewaskulyong B, Geater SL, Goksel T, Harputluoglu H, Artac M, Paydas S, Altundag O, Sencan O, Hicks J, Faivre-Finn C, Talbot T, Telivala B, Hussein M, McCLeod M, Slater D, Uyeki JVH, Waterhouse D, Chen FL, Hao Z, Kelly R, Morgensztern D, Page R, Spira AI, Awad M, Beck JT, Berg AR, Choksi J, Dorroh S, Goldman J, Iannotti N, Kubiak K, Rao S, Chaudhry A, Dalsania CJ, Farrell NJ, Hermann R, Kuzma CS, McIntyre KJ, Mitchell W, Rodriguez E, Sangal A, Smith DA, Zu K, Anderson IC, Behl D, Edenfield WJ, Ghazal H, Giaccone G, Hagenstad CT, Haigentz M Jr, Harper H, Henderson C, Hrinczenko B, Konduri K, Levine M, Martincic D, Pillai RN, Salamat M, Shtivelband M, Singh J, Socoteanu MP, Spigel D, Zorsky P, Ferrarotto R, Gomez J, Horn L, Kendall S, Lawler WE, Gandhi L, Zylla D, Assikis V, McCune S, Bailey S, Le AT, Mai K, Nguyen L.
- 덧글달기
- 이전글 [J Clin Oncol.] Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline.
- 다음글 [Ann Surg.] Surgical Management of Gallbladder Cancer: Simple Versus Extended Cholecystectomy and the Role of Adjuvant Therapy.







편집위원
NSCLC에서 기존의 표준치료인 definitive CCRT보다 치료성적의 향상을 보여준 practice changing 한 논문입니다.
덧글달기닫기2017-12-12 14:17:48
등록
편집위원2
수술이 불가능한 3기 비소세포성 폐암에서 항암방사선동시치료가 표준치료로 권고되고 있지만, 치료 성적은 최적이라고 할 수 없는 상태로, 결과 향상을 위한 여러가지 시도가 진행되고 있습니다. 그 중에서 항암방사선동시치료 후 Durvalumab 추가로 인한 progression free survival의 의미있는 향상을 보인 이번연구는 앞으로의 치료 패러다임을 변화시킬 중요한 연구로 생각됩니다.
덧글달기닫기2017-12-12 14:50:04
등록