(구)글로벌 핫이슈
방사선종양학
- [Lancet Oncol.] Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial.
National Institute of Oncology(Hungary) / Csaba Polgár*
- 출처
- Lancet Oncol.
- 등재일
- 2017 Feb
- 저널이슈번호
- 18(2):259-268. doi: 10.1016/S1470-2045(17)30011-6. Epub 2017 Jan 14.
- 내용
Abstract
BACKGROUND:
We previously confirmed the non-inferiority of accelerated partial breast irradiation (APBI) with interstitial brachytherapy in terms of local control and overall survival compared with whole-breast irradiation for patients with early-stage breast cancer who underwent breast-conserving surgery in a phase 3 randomised trial. Here, we present the 5-year late side-effects and cosmetic results of the trial.
METHODS:
We did this randomised, controlled, phase 3 trial at 16 centres in seven European countries. Women aged 40 years or older with stage 0-IIA breast cancer who underwent breast-conserving surgery with microscopically clear resection margins of at least 2 mm were randomly assigned 1:1, via an online interface, to receive either whole-breast irradiation of 50 Gy with a tumour-bed boost of 10 Gy or APBI with interstitial brachytherapy. Randomisation was stratified by study centre, menopausal status, and tumour type (invasive carcinoma vs ductal carcinoma in situ), with a block size of ten, according to an automated dynamic algorithm. Patients and investigators were not masked to treatment allocation. The primary endpoint of our initial analysis was ipsilateral local recurrence; here, we report the secondary endpoints of late side-effects and cosmesis. We analysed physician-scored late toxicities and patient-scored and physician-scored cosmetic results from the date of breast-conserving surgery to the date of onset of event. Analysis was done according to treatment received (as-treated population). This trial is registered with ClinicalTrials.gov, number NCT00402519.
FINDINGS:
Between April 20, 2004, and July 30, 2009, we randomly assigned 1328 women to receive either whole-breast irradiation (n=673) or APBI with interstitial brachytherapy (n=655); 1184 patients comprised the as-treated population (551 in the whole-breast irradiation group and 633 in the APBI group). At a median follow-up of 6·6 years (IQR 5·8-7·6), no patients had any grade 4 toxities, and three (<1%) of 484 patients in the APBI group and seven (2%) of 393 in the whole-breast irradiation group had grade 3 late skin toxicity (p=0·16). No patients in the APBI group and two (<1%) in the whole-breast irradiation group developed grade 3 late subcutaneous tissue toxicity (p=0·10). The cumulative incidence of any late side-effect of grade 2 or worse at 5 years was 27·0% (95% CI 23·0-30·9) in the whole-breast irradiation group versus 23·3% (19·9-26·8) in the APBI group (p=0·12). The cumulative incidence of grade 2-3 late skin toxicity at 5 years was 10·7% (95% CI 8·0-13·4) in the whole-breast irradiation group versus 6·9% (4·8-9·0) in the APBI group (difference -3·8%, 95% CI -7·2 to 0·4; p=0·020). The cumulative risk of grade 2-3 late subcutaneous tissue side-effects at 5 years was 9·7% (95% CI 7·1-12·3) in the whole-breast irradiation group versus 12·0% (9·4-14·7) in the APBI group (difference 2·4%; 95% CI -1·4 to 6·1; p=0·28). The cumulative incidence of grade 2-3 breast pain was 11·9% (95% CI 9·0-14·7) after whole-breast irradiation versus 8·4% (6·1-10·6) after APBI (difference -3·5%; 95% CI -7·1 to 0·1; p=0·074). At 5 years' follow-up, according to the patients' view, 413 (91%) of 454 patients had excellent to good cosmetic results in the whole-breast irradiation group versus 498 (92%) of 541 patients in the APBI group (p=0·62); when judged by the physicians, 408 (90%) of 454 patients and 503 (93%) of 542 patients, respectively, had excellent to good cosmetic results (p=0·12). No treatment-related deaths occurred, but six (15%) of 41 patients (three in each group) died from breast cancer, and 35 (85%) deaths (21 in the whole-breast irradiation group and 14 in the APBI group) were unrelated.
INTERPRETATION:
5-year toxicity profiles and cosmetic results were similar in patients treated with breast-conserving surgery followed by either APBI with interstitial brachytherapy or conventional whole-breast irradiation, with significantly fewer grade 2-3 late skin side-effects after APBI with interstitial brachytherapy. These findings provide further clinical evidence for the routine use of interstitial multicatheter brachytherapy-based APBI in the treatment of patients with low-risk breast cancer who opt for breast conservation.
FUNDING:
German Cancer Aid.
Implications of all the available evidence
To our knowledge, this trial is the first phase 3 study that shows similar late toxicity profiles and cosmetic outcomes, and significantly fewer late skin side-effects of APBI with interstitial brachytherapy compared with whole-breast irradiation for selected patients with early stage breast cancer. On the basis of our findings, APBI with interstitial brachytherapy can be regarded as a valid alternative treatment option after breast-conserving surgery, and can be offered for all patients with low-risk breast cancer as part of routine clinical practice.
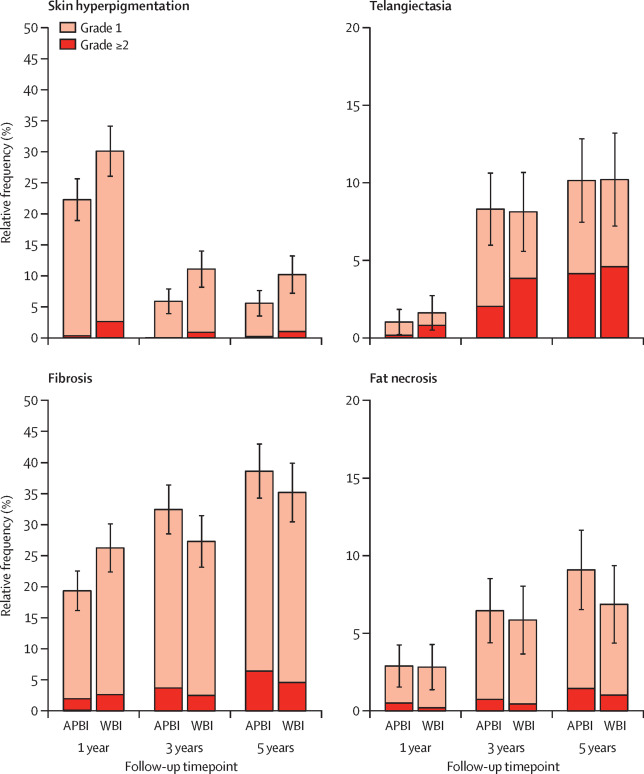
Figure 2.
Relative frequency of grade 1 and grade 2 or worse late side-effects
Error bars represent 95% CIs. APBI=accelerated partial breast irradiation. WBI=whole-breast irradiation.
Author information
Polgár C1, Ott OJ2, Hildebrandt G3, Kauer-Dorner D4, Knauerhase H5, Major T6, Lyczek J7, Guinot JL8, Dunst J9, Miguelez CG10, Slampa P11, Allgäuer M12, Lössl K13, Polat B14, Kovács G15, Fischedick AR16, Fietkau R17, Resch A4, Kulik A18, Arribas L8, Niehoff P19, Guedea F10, Schlamann A20, Pötter R4, Gall C21, Uter W21, Strnad V2; Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
1 Center of Radiotherapy, National Institute of Oncology, Budapest, Hungary. Electronic address: polgar@oncol.hu.
2 Department of Radiation Oncology, University Hospital Erlangen, Erlangen, Germany.
3 Department of Radiation Oncology, University Hospital Leipzig, Leipzig, Germany; Department of Radiation Oncology, University Hospital Rostock, Rostock, Germany.
4 Department of Radiation Oncology, University Hospital AKH Wien, Vienna, Austria.
5 Department of Radiation Oncology, University Hospital Rostock, Rostock, Germany.
6 Center of Radiotherapy, National Institute of Oncology, Budapest, Hungary.
7 Brachytherapy Department, Centrum Onkologii-Instytut im Marii Skłodowskiej, Warsaw, Poland; Podkarpacki Hospital Cancer Center Brzozów, Brzozów, Poland.
8 Department of Radiation Oncology, Valencian Institute of Oncology, Valencia, Spain.
9 Department of Radiation Oncology, University Hospital Kiel, Kiel, Germany.
10 Department of Radiation Oncology, Catalan Institute of Oncology, Barcelona, Spain.
11 Department of Radiation Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic.
12 Department of Radiation Oncology, Hospital Barmherzige Brüder, Regensburg, Germany.
13 Department of Radiation Oncology, University Hospital Bern, Inselspital, Switzerland.
14 Department of Radiation Oncology, University Hospital Würzburg, Würzburg, Germany.
15 Interdisciplinary Brachytherapy Unit, University of Lübeck/Universitätsklinikum Schleswig-Holstein Campus Lübeck, Lübeck, Germany.
16 Department of Radiation Oncology, Clemens Hospital, Münster, Germany.
17 Department of Radiation Oncology, University Hospital Erlangen, Erlangen, Germany; Department of Radiation Oncology, University Hospital Rostock, Rostock, Germany.
18 Brachytherapy Department, Centrum Onkologii-Instytut im Marii Skłodowskiej, Warsaw, Poland.
19 Department of Radiation Oncology, University Hospital Kiel, Kiel, Germany; Department of Radiotherapy, Sana Hospital Offenbach, Offenbach, Germany.
20 Department of Radiation Oncology, University Hospital Leipzig, Leipzig, Germany.
21 Department of Medical Informatics, Biometry and Epidemiology, University Erlangen-Nuremberg, Erlangen, Germany.
- 덧글달기
- 이전글 [Lancet Oncol.] A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study.
- 다음글 [Lancet Oncol.] Prevention of radiotherapy-induced neurocognitive dysfunction in survivors of paediatric brain tumours: the potential role of modern imaging and radiotherapy techniques.









