글로벌 연구동향
분자영상 및 방사화학
![[Cancer Biother Radiopharm.] Improved In Vivo Stability of Radioiodinated Rituximab Using an Iodination Linker for Radioimmunotherapy.](/enewspaper/upimages/admin_20161109154232_R.png) 2016년 11월호
2016년 11월호
[Cancer Biother Radiopharm.] Improved In Vivo Stability of Radioiodinated Rituximab Using an Iodination Linker for Radioimmunotherapy.KIRAMS / 김은정, 최태현*
- 출처
- Cancer Biother Radiopharm.
- 등재일
- 2016 Oct
- 저널이슈번호
- 31(8):287-294. Epub 2016 Sep 30.
- 내용
AbstractPURPOSE:
Directly radioiodinated [131I]-rituximab has been developed as a radioimmunotherapeutic agent in patients with CD20-positive B cell non-Hodgkin's lymphoma. However, there are concerns over its in vivo catabolism and deiodination. A novel radioiodination linker, N-(4-isothiocyanatobenzyl)-2-(3-(tributylstannyl)phenyl) acetamide (IBPA), was synthesized for the preparation of stable radioiodinated proteins.
METHODS:
The authors evaluated the potential of IBPA as a stable radioiodinated linker for rituximab. [125I]-IBPA was purified and conjugated with rituximab, and in vitro stability testing was performed in serum and liver microsomes. In vivo studies were performed after i.v. injection of [125I]-rituximab or [125I]-IBPA-rituximab to nude mice.
RESULTS:
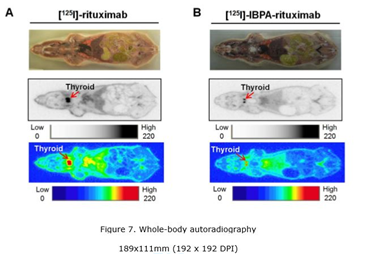
In in vitro studies, [125I]-IBPA-rituximab was stable in serum and liver microsomes. In static scans, high radioactivity was evident in the thyroid following injection of [125I]-rituximab, but low radioactivity was seen in the thyroid following injection of [125I]-IBPA-rituximab. In biodistribution studies, radioactivity uptake in thyroid glands of [125I]-IBPA-rituximab was decreased by approximately sevenfold compared to [125I]-rituximab. In pharmacokinetics, the half-life of [125I]-rituximab was shorter than that of [125I]-IBPA-rituximab in plasma of nude mice.
CONCLUSIONS:
The authors demonstrate that [125I]-IBPA-rituximab is more stable to metabolic deiodination in vivo than is [125I]-rituximab. Radioiodination of rituximab using IBPA is thus preferable to direct labeling in terms of in vivo stability.
Author information
Kim EJ1,2, Kim BS1,3, Choi DB1, Chi SG2, Choi TH3.
11 Korea Drug Development Platform using Radio-Isotope (KDePRI), Korea Institute of Radiological and Medical Sciences , Seoul, Korea.
22 School of Life Sciences and Biotechnology, Korea University , Seoul, Korea.
33 Department of Molecular Imaging, Korea Institute of Radiological and Medical Sciences , Seoul, Korea.
- 키워드
- CD20; N-(4-isothiocyanatobenzyl)-2-(3-(tributylstannyl)phenyl) acetamide (IBPA); deiodination; lymphoma; radioimmunotherapy; rituximab
- 연구소개
- I-131은 치료용방사성핵종중에서 국내 구입이 용이하고 비용효율이 좋다. 이러한 장점 때문에 한국원자력의학원에서는 직접표지 I-131 rituximab을 B 림프종 환자 치료에 사용하고 있다. 본 연구는 I-131을 rituximab에 표지시 기존의 직접표지방법에 비해 생체내 대사 안정성을 향상시키는 iodination linker를 사용하여 보다 적은 양의 방사능으로 치료효과를 높이고 독성을 낮출 수 있는 연구를 수행하였다.
- 덧글달기
- 이전글 [Cancer Biother Radiopharm.] Improved Pharmacokinetics Following PEGylation and Dimerization of a c(RGD-ACH-K) Conjugate Used for Tumor Positron Emission Tomography Imaging.
- 다음글 [Theranostics.] RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy







