글로벌 연구동향
핵의학
- 2024년 04월호
[J Nanobiotechnology .] Development of finely tuned liposome nanoplatform for macrophage depletion서울의대 / 최태현, 유란지, 임형준*, 이윤상*
- 출처
- J Nanobiotechnology .
- 등재일
- 2024 Feb 29
- 저널이슈번호
- 22(1):83. doi: 10.1186/s12951-024-02325-7.
- 내용
Abstract
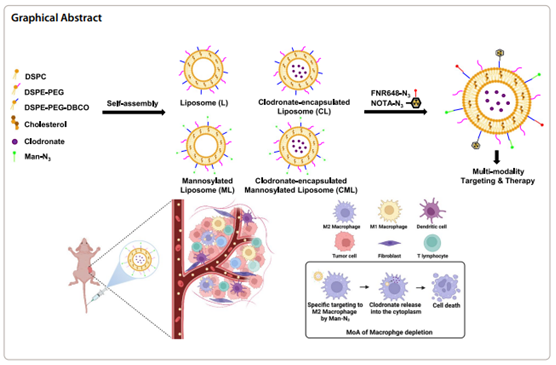
Background: Immunotherapy with clodronate-encapsulated liposomes, which induce macrophage depletion, has been studied extensively. However, previously reported liposomal formulation-based drugs (Clodrosome® and m-Clodrosome®) are limited by their inconsistent size and therapeutic efficacy. Thus, we aimed to achieve consistent therapeutic effects by effectively depleting macrophages with uniform-sized liposomes.Results: We developed four types of click chemistry-based liposome nanoplatforms that were uniformly sized and encapsulated with clodronate, for effective macrophage depletion, followed by conjugation with Man-N3 and radiolabeling. Functionalization with Man-N3 improves the specific targeting of M2 macrophages, and radioisotope labeling enables in vivo imaging of the liposome nanoplatforms. The functionalized liposome nanoplatforms are stable under physiological conditions. The difference in the biodistribution of the four liposome nanoplatforms in vivo were recorded using positron emission tomography imaging. Among the four platforms, the clodronate-encapsulated mannosylated liposome effectively depleted M2 macrophages in the normal liver and tumor microenvironment ex vivo compared to that by Clodrosome® and m-Clodrosome®.
Conclusion: The newly-developed liposome nanoplatform, with finely tuned size control, high in vivo stability, and excellent ex vivo M2 macrophage targeting and depletion effects, is a promising macrophage-depleting agent.
Affiliations
Tae Hyeon Choi # 1 2, Ran Ji Yoo # 1 3 4, Ji Yong Park 1 5, Ji Yoon Kim 1 5, Young Chan Ann 1 6, Jeongbin Park 2, Jin Sil Kim 1, Kyuwan Kim 1, Yu Jin Shin 1 7 8, Yong Jin Lee 9, Kyo Chul Lee 9, Jisu Park 7 8 10, Hyewon Chung 7 8 10, Seung Hyeok Seok 7 8 10, Hyung-Jun Im 11 12 13, Yun-Sang Lee 14 15 16 17 18
1Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, South Korea.
2Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, 08826, South Korea.
3Department of Nuclear Medicine, Seoul National University Hospital, 101 Daehak-Ro, Jongno-Gu, Seoul, South Korea.
4Biomedical Research Institute, Seoul National University Hospital, Seoul, South Korea.
5Institute of Radiation Medicine, Medical Research Center, Seoul National University College of Medicine, Seoul, South Korea.
6School of Dentistry, Seoul National University, Seoul, South Korea.
7Cancer Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
8Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, South Korea.
9Division of Applied RI, Korea Institute of Radiological and Medical Sciences (KIRAMS), Seoul, South Korea.
10Department of Microbiology and Immunology, and Institute of Endemic Disease, Seoul National University College of Medicine, Seoul, South Korea.
11Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, 08826, South Korea. iiihjjj@snu.ac.kr.
12Institute of Radiation Medicine, Medical Research Center, Seoul National University College of Medicine, Seoul, South Korea. iiihjjj@snu.ac.kr.
13Cancer Research Institute, Seoul National University College of Medicine, Seoul, South Korea. iiihjjj@snu.ac.kr.
14Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, South Korea. wonza43@snu.ac.kr.
15Department of Nuclear Medicine, Seoul National University Hospital, 101 Daehak-Ro, Jongno-Gu, Seoul, South Korea. wonza43@snu.ac.kr.
16Institute of Radiation Medicine, Medical Research Center, Seoul National University College of Medicine, Seoul, South Korea. wonza43@snu.ac.kr.
17Cancer Research Institute, Seoul National University College of Medicine, Seoul, South Korea. wonza43@snu.ac.kr.
18Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, South Korea. wonza43@snu.ac.kr.
#Contributed equally.
- 키워드
- Click chemistry; Clodronate; Liposome; Macrophage; Molecular imaging.
- 연구소개
- 종양 내 M2 마크로파지는 T세포 활성화를 억제하여 종양 성장을 촉진하고 면역관문 억제제의 효과를 반감시킬 뿐만 아니라 내성을 유도하는 면역억제성 마크로파지로 존재함. 따라서 면역억제성 M2 마크로파지에 선택적으로 표적되는 약물전달체의 개발이 중요함. 이에 본 연구에서는 클릭 화학을 기반으로 정교하게 조절된 리포좀 표면 만노실레이션을 통해 종양미세환경에서 M2 마크로파지를 표적하는 약물전달체 개발하였음. 이 약물전달체에 마크로파지를 사멸시킬 수 있는 클로드로네이트를 담지하여 마우스 암 모델의 치료 실험을 수행하였을 때, 종양 내 M2 마크로파지의 효과적인 사멸 효과를 확인하였음. 따라서 향 후 T세포 활성화 및 종양 감소 효과를 기대할 수 있는 나노 면역 치료제로 개발을 기대해 볼 수 있음.
- 덧글달기








편집위원
Macrophage는 다양한 질환의 발생 및 진행에 영향을 미치며, Macrophage의 M2 phenotype은 악성종양의 진행을 증진시키는 것으로 알려져 있음. Macrophage 제거는 악성종양의 진행을 억제하는 역할을 할 수 있음. 해당연구는 liposome nanoplatform을 이용하여 Macrophage를 제거하는 방법 중 크기 조절을 통해 Macrophage 제거능을 높이고 안정화시키는 연구임. 대식세포 조절을 이용하여 질환치료 개발에 관심을 가진 연구자에게 흥미를 끌 것 기초연구로 생각됨.
덧글달기닫기2024-03-29 15:25:48
등록