글로벌 연구동향
방사선종양학
- 2024년 11월호
[Int J Radiat Oncol Biol Phys .] Safety and Efficacy of HL301 In Radiation Pneumonitis in Patients With Unresectable Non-Small Cell Lung Cancer Receiving Curative Concurrent Chemoradiotherapy: A Multicenter, Randomized, Double-Blinded, Placebo-Controlled, Phase 2a Clinical Trial연세의대 / 김경환, 조재호*
- 출처
- Int J Radiat Oncol Biol Phys .
- 등재일
- 2024 Oct 1
- 저널이슈번호
- 120(2):432-438.
- 내용
Abstract
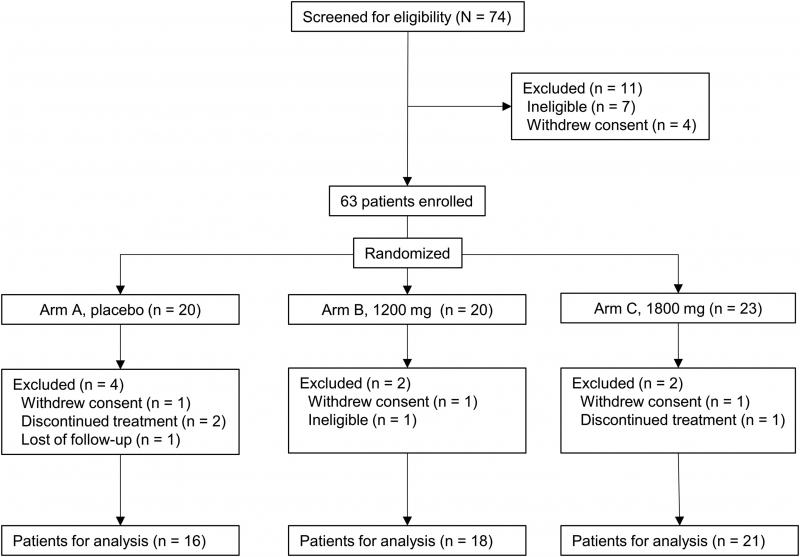
Purpose: We aimed to investigate the safety and efficacy of HL301, a standardized combination product of 7 medicinal plants, in radiation pneumonitis in patients with unresectable non-small cell lung cancer undergoing curative concurrent chemoradiotherapy.Methods and materials: The target accrual was 87 and a total of 63 patients were enrolled due to poor accrual rate. We randomly assigned the 63 patients to receive a placebo (arm A), or 1200 mg HL301 (arm B), or 1800 mg HL301 (arm C). Patients received weekly paclitaxel and carboplatin concurrently with intensity-modulated radiation therapy at 60 to 66 Gy in conventional fractionation. Durvalumab was administered as a maintenance treatment according to standard clinical practice. HL301 was administered orally, daily for 12 weeks. The primary endpoint was incidence of grade ≥2 radiation pneumonitis at 24 weeks postchemoradiotherapy.
Results: The baseline characteristics of the patients were well balanced. The drug was tolerable with a compliance rate of 86.6%, 86.2%, and 88.8% in arms A, B, and C, respectively (P = .874). None of the patients experienced severe drug-related adverse events. No significant difference in the rate of adverse events were observed between the treatment arms. The incidence of grade ≥2 radiation pneumonitis at 24 weeks postchemoradiotherapy was 37.5% (95% CI, 18.5%-61.4%), 55.6% (95% CI, 33.7%-75.4%), and 52.4% (95% CI, 32.4%-71.7%) in arms A, B, and C, respectively (P = .535).
Conclusions: This is the first exploratory clinical trial to test the safety and efficacy of HL301 in patients with non-small cell lung cancer. Safety and feasibility of HL301 were established but no signals of efficacy in reducing radiation pneumonitis was observed in this dose level.
Affiliations
Kyung Hwan Kim 1, Nahyun Kang 2, Si Yeol Song 3, Hak Jae Kim 4, Yeon-Sil Kim 5, Mi Jin Oh 6, Jaeho Cho 7
1Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, Seoul, Republic of Korea.
2School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea; R&D Center, Hanlim Pharm. Co, Ltd, Seoul, Republic of Korea.
3Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
4Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea.
5Department of Radiation Oncology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Republic of Korea.
6R&D Center, Hanlim Pharm. Co, Ltd, Seoul, Republic of Korea.
7Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, Seoul, Republic of Korea. Electronic address: jjhmd@yuhs.ac.
- 연구소개
- 본 연구는 국내연구진과 제약사 연구소가 협력하여 기초중개부터 시작하여 한국식약처에서 임상IIA상 승인을 받은 천연물 추출물 (HL-301)로 폐암 환자에서 흔히 발생하는 방사선폐손상 (폐렴, 폐섬유화)를 극복해보고자 시행된 임상시험에 대한 결과 보고입니다. 본 연구는 탐색연구로 HL301의 안전성과 적용 가능성을 입증하였습니다. 약물 용량 및 환자수를 최적화 하여 추후 임상 IIb 혹은 III상 에서 약물의 효능을 확인할 필요가 있습니다.
- 덧글달기
- 이전글 [Cancer Res Treat .] Prognostic Significance of Bulky Nodal Disease in Anal Cancer Management: A Multi-institutional Study
- 다음글 [Neurosurgery .] Optimizing Recurrent Glioblastoma Salvage Treatment: A Multicenter Study Integrating Genetic Biomarkers From the Korean Radiation Oncology Group (21-02)








편집위원
우리나라에서 전통적으로 쓰는 기침과 천식이 오래도록 낫지 않는 것을 치료하는 처방제의 성분인 HL301의 안전성과 효율성을 화학적방사선 치료를 받고 있는 폐암 환자들 중 방사선 폐렴을 보유한 환자에서 연구를 시도한 첫 임상 2상의 연구결과이며, 방사선폐렴을 줄이는 효율성 면에서는 차이가 없다는 연구결과를 보고함.
덧글달기닫기2024-10-18 14:41:19
등록