(구)글로벌 핫이슈
방사선종양학
- [J Clin Oncol.] Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial.
Erasmus MC–University Medical Center / Bo Jan Noordman*
- 출처
- J Clin Oncol.
- 등재일
- 2018 Jan 20
- 저널이슈번호
- 36(3):268-275.
- 내용
Abstract
Purpose
To compare pre-agreed health-related quality of life (HRQOL) domains in patients with esophageal or junctional cancer who received neoadjuvant chemoradiotherapy (nCRT) followed by surgery or surgery alone. Secondary aims were to examine the effect of nCRT on HRQOL before surgery and the effect of surgery on HRQOL.
Patients and Methods
Patients were randomly assigned to nCRT (carboplatin plus paclitaxel with concurrent 41.4-Gy radiotherapy) followed by surgery or surgery alone. HRQOL was measured using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (QLQ-C30) and -Oesophageal CancerModule (QLQ-OES24) questionnaires pretreatment and at 3, 6, 9, and 12 months postoperatively. The nCRT group also received preoperative questionnaires. Physical functioning (PF; QLQ-C30) and eating problems (EA; QLQ-OES24) were chosen as predefined primary end points. Predefined secondary end points were global QOL (GQOL; QLQ-C30), fatigue (FA; QLQ-C30), and emotional problems (EM; QLQ-OES24).
Results
A total of 363 patients were analyzed. No statistically significant differences in postoperative HRQOL were found between treatment groups. In the nCRT group, PF, EA, GQOL, FA, and EM scores deteriorated 1 week after nCRT (Cohen's d: -0.93, P < .001; 0.47, P < .001; -0.84, P < .001; 1.45, P < .001; and 0.32, P = .001, respectively). In both treatment groups, all end points declined 3 months postoperatively compared with baseline (Cohen's d: -1.00, 0.33, -0.47, -0.34, and 0.33, respectively; all P < .001), followed by a continuous gradual improvement. EA, GQOL, and EM were restored to baseline levels during follow-up, whereas PF and FA remained impaired 1 year postoperatively (Cohen's d: 0.52 and -0.53, respectively; both P < .001).
Conclusion
Although HRQOL declined during nCRT, no effect of nCRT was apparent on postoperative HRQOL compared with surgery alone. In addition to the improvement in survival, these findings support the view that nCRT according to the Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study-regimen can be regarded as a standard of care.
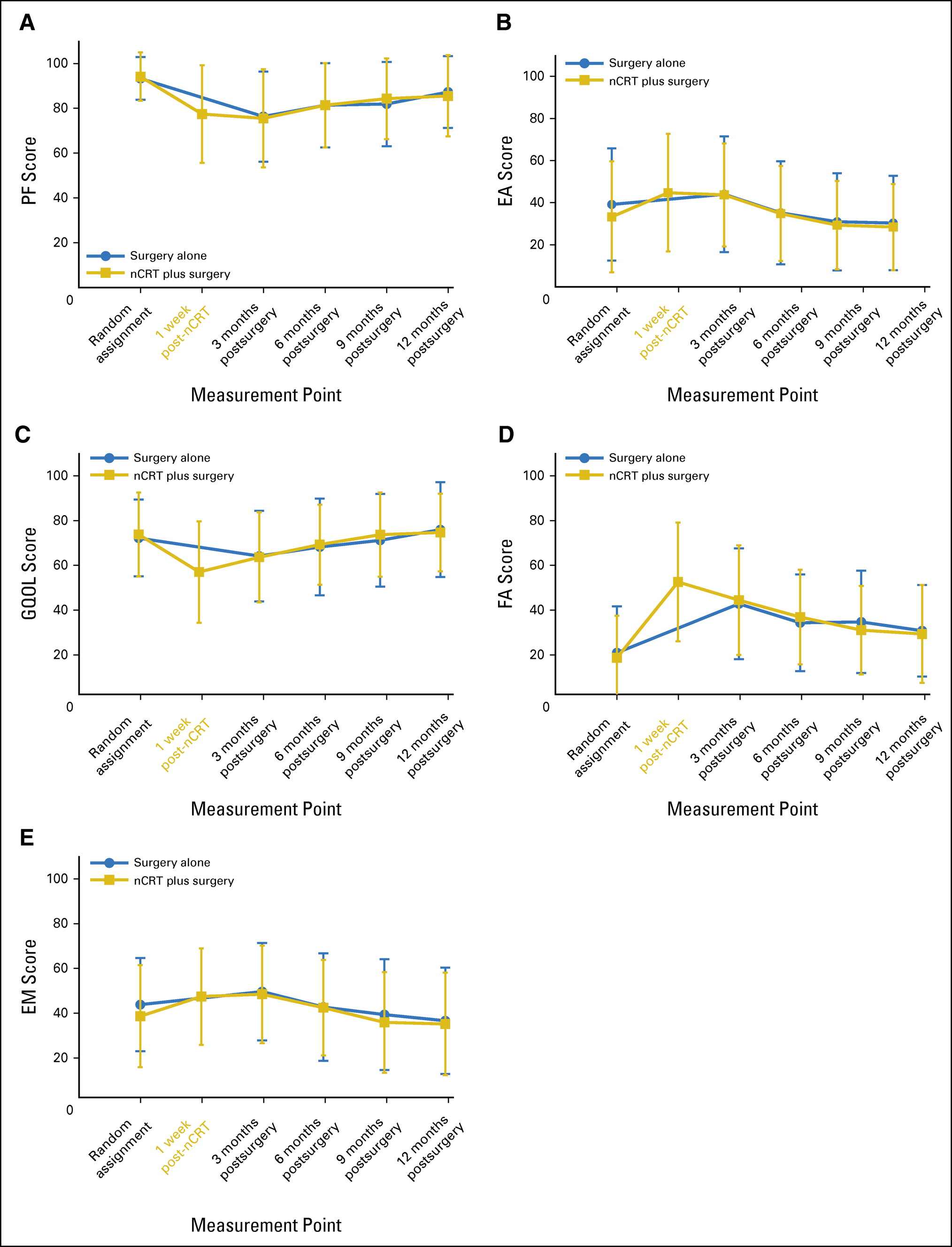
Fig 2.Mean scores with standard deviations for primary end points (A) physical functioning (PF) and (B) eating problems (EA) and secondary end points (C) global quality of life (GQOL), (D) fatigue (FA), and (E) emotional problems (EM) according to treatment group. nCRT, neoadjuvant chemoradiotherapy.
Author information
Noordman BJ1, Verdam MGE1, Lagarde SM1, Hulshof MCCM1, van Hagen P1, van Berge Henegouwen MI1, Wijnhoven BPL1, van Laarhoven HWM1, Nieuwenhuijzen GAP1, Hospers GAP1, Bonenkamp JJ1, Cuesta MA1, Blaisse RJB1, Busch OR1, Ten Kate FJW1, Creemers GM1, Punt CJA1, Plukker JTM1, Verheul HMW1, Spillenaar Bilgen EJ1, van Dekken H1, van der Sangen MJC1, Rozema T1, Biermann K1, Beukema JC1, Piet AHM1, van Rij CM1, Reinders JG1, Tilanus HW1, Steyerberg EW1, van der Gaast A1, Sprangers MAG1, van Lanschot JJB1.
1 Bo Jan Noordman, Sjoerd M. Lagarde, Pieter van Hagen, Bas P.L. Wijnhoven, Fiebo J.W. ten Kate, Katharina Biermann, Caroline M. van Rij, Hugo W. Tilanus, Ewout W. Steyerberg, Ate van der Gaast, and J. Jan B. van Lanschot, Erasmus MC-University Medical Center Rotterdam; Mathilde G.E. Verdam, Maarten C.C.M. Hulshof, Mark I. van Berge Henegouwen, Hanneke W.M. van Laarhoven, Olivier R. Busch, Fiebo J.W. ten Kate, Cornelis J.A. Punt, and Mirjam A.G. Sprangers, Academic Medical Center; Miguel A. Cuesta, Henk M.W. Verheul, and Anna H.M. Piet, Vrije Universiteit Medical Center; Herman van Dekken, St Lucas Andreas Hospital, Amsterdam; Grard A.P. Nieuwenhuijzen, Geert-Jan M. Creemers, and Maurice J.C. van der Sangen, Catharina Hospital, Eindhoven; Geke A.P. Hospers, John Th.M. Plukker, and Jannet C. Beukema, University Medical Center Groningen, Groningen; Johannes J. Bonenkamp, Cornelis J.A. Punt, and Tom Rozema, Radboud University Nijmegen Medical Center, Nijmegen; Reinoud J.B. Blaisse and Ernst J. Spillenaar Bilgen, Rijnstate Hospital; Janny G. Reinders, Arnhem Radiotherapeutic Institute, Arnhem; and Tom Rozema, Verbeeten Institute, Tilburg, the Netherlands.
- 덧글달기









