글로벌 연구동향
핵의학
- [EJNMMI Res.] A preliminary clinical trial to evaluate 64 Cu-NOTA-Trastuzumab as a positron emission tomography imaging agent in patients with breast cancer
KIRAMS / 이인기, 임일한*, 노우철*
- 출처
- EJNMMI Res.
- 등재일
- 2021 Jan 21
- 저널이슈번호
- 11(1):8. doi: 10.1186/s13550-021-00746-1.
- 내용
Abstract
Background: The purpose of this study was to evaluate both the biodistribution and safety of 64Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)-Trastuzumab, a novel 64Cu-labeled positron emission tomography (PET) tracer for human epidermal growth factor receptor 2 (HER2) in patients with breast cancer.Methods: PET images at 1, 24, and 48 h after 296 MBq of 64Cu-NOTA-Trastuzumab injection were obtained from seven patients with breast cancer. Both the primary tumors' and metastatic lesions' maximum standardized uptake value (SUVmax) was evaluated. The mean SUVmax (SUVmean) was evaluated in the other organs, including the blood pool, liver, kidney, muscle, spleen, bladder, and the lungs, as well as the bones. Moreover, the internal radiation dosimetry was calculated using the OLINDA/EXM software. Safety was assessed based on feedback regarding adverse reactions and safety-related issues within 1 month after 64Cu-NOTA-Trastuzumab administration.
Results: 64Cu-NOTA-Trastuzumab PET images showed that the overall SUVmean values in each organ negatively correlated with time. The liver's average SUVmean values were measured at 5.3 ± 0.7, 4.8 ± 0.6, and 4.4 ± 0.5 on 1 h, 24 h, and 48 h after injection, respectively. The average SUVmean blood values were measured at 13.1 ± 0.9, 9.1 ± 1.2, and 7.1 ± 1.9 on 1 h, 24 h, and 48 h after injection, respectively. The SUVmax of HER2-positive tumors was relatively higher than HER2-negative tumors (8.6 ± 5.1 and 5.2 ± 2.8 on 48 h after injection, respectively). Tumor-to-background ratios were higher in the HER2-positive tumors than in the HER2-negative tumors. No adverse events related to 64Cu-NOTA-Trastuzumab were reported. The calculated effective dose with a 296 MBq injection of 64Cu-NOTA-Trastuzumab was 2.96 mSv. The highest absorbed dose was observed in the liver (0.076 mGy/MBq), followed by the spleen (0.063 mGy/MBq), kidney (0.044 mGy/MBq), and heart wall (0.044 mGy/MBq).
Conclusions: 64Cu-NOTA-Trastuzumab showed a specific uptake at the HER2-expressing tumors, thus making it a feasible and safe monitoring tool of HER2 tumor status in patients with breast cancer.
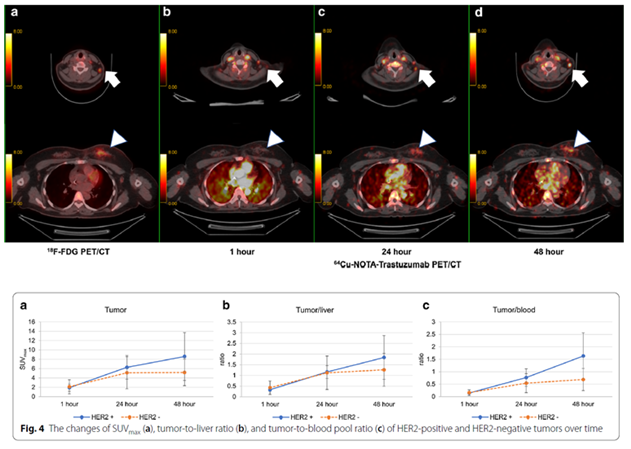
시간 경과에 따른 HER2 발현 PET/CT 영상 및 방사성의약품 섭취 차이를 나타내는 그래프
방사성의약품(Cu-64-NOTA-trastuzumab) 섭취 정도를 지표로 표기한 최대 표준화 섭취계수(SUVmax)가 HER2 양성 종양은 평균 8.6, HER2 음성 종양은 5.2로 낮게 측정됨
Affiliations
Inki Lee 1 , Ilhan Lim # 2 , Byung Hyun Byun 1 , Byung Il Kim 1 , Chang Woon Choi 1 , Sang-Keun Woo 3 , Kwang Il Kim 3 , Kyo Chul Lee 3 , Joo Hyun Kang 3 , Min-Ki Seong 4 , Hyun-Ah Kim 4 , Woo Chul Noh # 5 , Sang Moo Lim 1
1 Department of Nuclear Medicine, Korea Cancer Centre Hospital, Korea Institute of Radiological and Medical Sciences, 75, Nowon-ro, Nowon-gu, Seoul, Korea.
2 Department of Nuclear Medicine, Korea Cancer Centre Hospital, Korea Institute of Radiological and Medical Sciences, 75, Nowon-ro, Nowon-gu, Seoul, Korea. ilhan@kcch.re.kr.
3 Division of Applied RI, Research Institute of Radiological and Medical Sciences, Korea Institutes of Radiological and Medical Sciences, Seoul, Korea.
4 Department of Surgery, Korea Cancer Centre Hospital, Korea Institutes of Radiological and Medical Sciences, 75, Nowon-ro, Nowon-gu, Seoul, Korea.
5 Department of Surgery, Korea Cancer Centre Hospital, Korea Institutes of Radiological and Medical Sciences, 75, Nowon-ro, Nowon-gu, Seoul, Korea. nohwoo@kirams.re.kr.
# Contributed equally.
- 키워드
- 64Cu-NOTA-Trastuzumab; Breast cancer; Computed tomography; HER-2; Positron emission tomography.
- 연구소개
- 연구팀은 7명의 유방암 환자를 대상으로 HER2를 표적으로 하는 방사성의약품(Cu-64-NOTA-trastuzumab)*을 주사하고, PET/CT 영상으로 시간 경과에 따른 HER2의 발현 여부를 정량적으로 분석 평가했다. 먼저, 방사성의약품(Cu-64-NOTA-trastuzumab)을 유방암 환자군에 주사 하고 1시간, 24시간, 48시간째 각 시점의 PET/CT 영상을 촬영하였고, 분석 결과, 방사성의약품 섭취 정도를 지표로 표기한 최대 표준화 섭취계수(SUVmax)가 HER2 양성 종양에서는 평균 8.6, HER2 음성 종양은 5.2로 낮게 측정됐다. 이번 연구결과로 연구팀은 HER2가 있는 양성 종양에 방사성의약품(Cu-64-NOTA-trastuzumab)이 더 많이 축적되는 것을 PET/CT 영상으로 확인하였고, 환자군에서 이상 증상이 나타나지 않아 방사성의약품(Cu-64-NOTA-trastuzumab)이 HER2 발현 여부 진단에 있어 뛰어난 효용성과 안전성이 있음을 확인했다. 유방암 환자의 HER2 유전자 진단의 번거로움을 최소화하고 정밀 진단으로 치료 효과를 높여 생존율 향상에 기여하고, 앞으로 유방암 뿐 아니라 다른 암종의 표적항암제 및 면역항암제 치료에 활용할 수 있기를 바란다.
- 덧글달기
- 이전글 [Front Psychiatry.] PET Hypometabolism of the Prefrontal-Cingulate Cortices in Internet Gaming Disorder
- 다음글 [Ann Nucl Med.] Immune microenvironment of the gene signature reflecting the standardised uptake value on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography in head and neck squamous cell carcinoma










편집위원
HER2 tumor status를 영상을 통해 비침습적으로 평가할 수 있어 임상에 쉽게 적용가능할 것으로 생각됩니다.
2021-03-08 10:47:07