글로벌 연구동향
방사선종양학
- [Int J Radiat Oncol Biol Phys.] A Randomized Phase 2 Trial of Consolidation Chemotherapy After Preoperative Chemoradiation Therapy Versus Chemoradiation Therapy Alone for Locally Advanced Rectal Cancer: KCSG CO 14-03.
국립암센터 / 김선영, 백지연*
- 출처
- Int J Radiat Oncol Biol Phys.
- 등재일
- 2018 Jul 15
- 저널이슈번호
- 101(4):889-899. doi: 10.1016/j.ijrobp.2018.04.013. Epub 2018 Apr 12.
- 내용
Abstract
PURPOSE:
Preoperative chemoradiation therapy (CRT) followed by total mesorectal excision (TME) in locally advanced rectal cancer is the standard of care. To date, the role of consolidation chemotherapy after CRT has rarely been addressed through randomized trials. This study aimed to evaluate the efficacy of CRT followed by consolidation chemotherapy compared with CRT alone.METHODS AND MATERIALS:
This study enrolled patients with adenocarcinoma of the rectum and cT3 or cT4 disease with any N category and no metastasis. In arm A (control arm), we planned CRT (50.4 Gy in 28 fractions) with capecitabine followed by TME. In arm B, 2 cycles of capecitabine and oxaliplatin were administered 1 week after the completion of CRT before TME (capecitabine, 1700 mg/m2 per day from day 1 to 14, and oxaliplatin, 100 mg/m2 on day 1, every 3 weeks). The downstaging rate (the proportion of ypT0 to ypT2 and ypN0M0) was the primary endpoint, which was to be tested with a 1-sided type I error of 15% and with 85% power.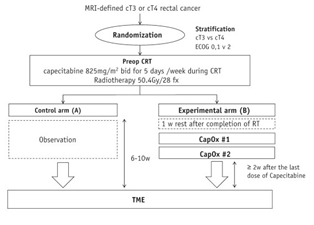
RESULTS:
From September 2014 to February 2016, 110 patients (56 in arm A and 54 in arm B) were randomized and 108 (55 in arm A and 53 in arm B) started CRT. TME was conducted per protocol in 96 patients (52 in arm A and 44 in arm B). In arms A and B, downstaging was achieved in 21.2% and 36.4% (P = .077), respectively, and the pathologic complete response rate was 5.8% and 13.6% (P = .167), respectively. Grade ≥3 adverse events occurred in 3.6% of patients in arm A and 9.4% of patients in arm B during the preoperative treatment phase and in 1.9% and 9.0%, respectively, during the postoperative recovery phase.CONCLUSIONS:
Consolidation chemotherapy with 2 cycles of capecitabine and oxaliplatin demonstrated a marginal improvement in the downstaging rate. However, a phase 3 trial of this strategy is discouraged because of the high dropout rate and safety issues.
Author informationKim SY1, Joo J2, Kim TW1, Hong YS1, Kim JE1, Hwang IG3, Kim BG4, Lee KW5, Kim JW5, Oh HS6, Ahn JB7, Zang DY8, Kim DY9, Oh JH9, Baek JY10.
1
Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
2
Biometric Research Branch, Research Institute and Hospital, National Cancer Center, Goyang, Republic of Korea.
3
Division of Hematology/Oncology, Department of Internal Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Republic of Korea.
4
Department of Surgery, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Republic of Korea.
5
Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Republic of Korea.
6
Department of Internal Medicine, Gangneung Asan Medical Center, University of Ulsan College of Medicine, Gangneung, Republic of Korea.
7
Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea.
8
Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Anyang, Republic of Korea.
9
Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Republic of Korea.
10
Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Republic of Korea. Electronic address: jbaek@ncc.re.kr.
- 덧글달기
- 이전글 [Int J Radiat Oncol Biol Phys.] Total Mesorectal Excision Versus Local Excision After Preoperative Chemoradiotherapy in Rectal Cancer With Lymph Node Metastasis: A Propensity Score-Matched Analysis.
- 다음글 [Radiat Oncol.] Feasibility of stereotactic radiotherapy for lung lesions and conventional radiotherapy for nodal areas in primary lung malignancies.









