(구)글로벌 핫이슈
방사선종양학
- [Lancet.] 새롭게 진단된 전이성 전립선암에서 원발종양 부위 방사선치료 (STAMPEDE): 무작위배정 3상연구 Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial.
MRC Clinical Trials Unit at UCL, Royal Marsden Hospital / Matthew R Sydes*, Chris C Parker*
- 출처
- Lancet.
- 등재일
- 2018 Dec 1
- 저널이슈번호
- 392(10162):2353-2366. doi: 10.1016/S0140-6736(18)32486-3. Epub 2018 Oct 21.
- 내용
Abstract
BACKGROUND:
Based on previous findings, we hypothesised that radiotherapy to the prostate would improve overall survival in men with metastatic prostate cancer, and that the benefit would be greatest in patients with a low metastatic burden. We aimed to compare standard of care for metastatic prostate cancer, with and without radiotherapy.METHODS:
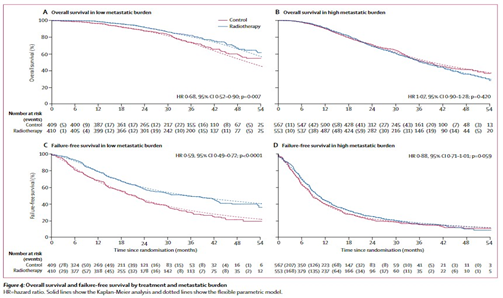
We did a randomised controlled phase 3 trial at 117 hospitals in Switzerland and the UK. Eligible patients had newly diagnosed metastatic prostate cancer. We randomly allocated patients open-label in a 1:1 ratio to standard of care (control group) or standard of care and radiotherapy (radiotherapy group). Randomisation was stratified by hospital, age at randomisation, nodal involvement, WHO performance status, planned androgen deprivation therapy, planned docetaxel use (from December, 2015), and regular aspirin or non-steroidal anti-inflammatory drug use. Standard of care was lifelong androgen deprivation therapy, with up-front docetaxel permitted from December, 2015. Men allocated radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomisation. The primary outcome was overall survival, measured as the number of deaths; this analysis had 90% power with a one-sided α of 2·5% for a hazard ratio (HR) of 0·75. Secondary outcomes were failure-free survival, progression-free survival, metastatic progression-free survival, prostate cancer-specific survival, and symptomatic local event-free survival. Analyses used Cox proportional hazards and flexible parametric models, adjusted for stratification factors. The primary outcome analysis was by intention to treat. Two prespecified subgroup analyses tested the effects of prostate radiotherapy by baseline metastatic burden and radiotherapy schedule. This trial is registered with ClinicalTrials.gov, number NCT00268476.FINDINGS:
Between Jan 22, 2013, and Sept 2, 2016, 2061 men underwent randomisation, 1029 were allocated the control and 1032 radiotherapy. Allocated groups were balanced, with a median age of 68 years (IQR 63-73) and median amount of prostate-specific antigen of 97 ng/mL (33-315). 367 (18%) patients received early docetaxel. 1082 (52%) participants nominated the daily radiotherapy schedule before randomisation and 979 (48%) the weekly schedule. 819 (40%) men had a low metastatic burden, 1120 (54%) had a high metastatic burden, and the metastatic burden was unknown for 122 (6%). Radiotherapy improved failure-free survival (HR 0·76, 95% CI 0·68-0·84; p<0·0001) but not overall survival (0·92, 0·80-1·06; p=0·266). Radiotherapy was well tolerated, with 48 (5%) adverse events (Radiation Therapy Oncology Group grade 3-4) reported during radiotherapy and 37 (4%) after radiotherapy. The proportion reporting at least one severe adverse event (Common Terminology Criteria for Adverse Events grade 3 or worse) was similar by treatment group in the safety population (398 [38%] with control and 380 [39%] with radiotherapy).INTERPRETATION:
Radiotherapy to the prostate did not improve overall survival for unselected patients with newly diagnosed metastatic prostate cancer.Implications of all the available evidence
Evidence suggests that prostate radiotherapy improves overall survival for men with metastatic prostate cancer who have a low metastatic burden, but not for unselected patients. Prostate radiotherapy should be a standard treatment option for men with newly diagnosed disease with a low metastatic burden.
Author informationParker CC1, James ND2, Brawley CD3, Clarke NW4, Hoyle AP4, Ali A5, Ritchie AWS3, Attard G6, Chowdhury S7, Cross W8, Dearnaley DP9, Gillessen S10, Gilson C3, Jones RJ11, Langley RE3, Malik ZI12, Mason MD13, Matheson D14, Millman R3, Russell JM15, Thalmann GN16, Amos CL3, Alonzi R17, Bahl A18, Birtle A19, Din O20, Douis H21, Eswar C12, Gale J22, Gannon MR23, Jonnada S24, Khaksar S25, Lester JF26, O'Sullivan JM27, Parikh OA28, Pedley ID29, Pudney DM30, Sheehan DJ31, Srihari NN32, Tran ATH33, Parmar MKB3, Sydes MR34; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators.
1
Academic Urology Unit, Royal Marsden Hospital, London, UK; Institute of Cancer Research, London, UK. Electronic address: chris.parker@icr.ac.uk.
2
Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, UK.
3
Medical Research Council (MRC) Clinical Trials Unit at University College London (UCL), London, UK.
4
Genito-Urinary Cancer Research Group, Department of Surgery, The Christie Hospital, Manchester, UK; Department of Urology, Salford Royal Hospitals, Manchester, UK.
5
Genito-Urinary Cancer Research Group, Department of Surgery, The Christie Hospital, Manchester, UK; The FASTMAN/Genito-Urinary Cancer Research Groups, Division of Cancer Sciences, and Belfast-Manchester Movember Centre of Excellence, Manchester Cancer Research Centre, University of Manchester, Manchester, UK.
6
UCL Cancer Institute, UCL, London, UK.
7
Department of Medical Oncology, Guy's & St Thomas' NHS Foundation Trust, London, UK.
8
Department of Urology, St James University Hospital, Leeds, UK.
9
Academic Urology Unit, Royal Marsden Hospital, London, UK; Institute of Cancer Research, London, UK.
10
Division of Cancer Sciences, University of Manchester and the Christie, Manchester, UK; Division of Oncology and Haematology, Kantonsspital, St Gallen, Switzerland.
11
Beatson West of Scotland Cancer Centre, University of Glasgow, Glasgow, UK.
12
The Clatterbridge Cancer Centre NHS Foundation Trust, Liverpool, UK.
13
Division of Cancer & Genetics, School of Medicine, Cardiff University, Cardiff, UK.
14
Faculty of Education, Health and Wellbeing, University of Wolverhampton, Wolverhampton, UK.
15
Institute of Cancer Sciences, University of Glasgow, Glasgow, UK.
16
Department of Urology, University Hospital, Inselspital, Bern, Switzerland.
17
Mount Vernon Cancer Centre, London, UK.
18
Bristol Haematology and Oncology Centre, Bristol, UK.
19
Rosemere Cancer Centre, Lancashire Teaching Hospitals, Preston, UK; School of Cancer Sciences, University of Manchester, Manchester, UK.
20
Department of Clinical Oncology, Weston Park Cancer Centre, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
21
Department of Radiology, University Hospital Birmingham NHS Foundation Trust, Birmingham, UK.
22
Portsmouth Oncology Centre, Queen Alexandra Hospital, Portsmouth, UK.
23
Department of Health Services Research, London School of Hygiene & Tropical Medicine, London, UK.
24
Department of Oncology, Gloucestershire Hospitals NHS Foundation Trust, Gloucester, UK.
25
St Luke's Cancer Centre, Royal Surrey County Hospital, Guildford, UK.
26
Velindre Cancer Centre, Cardiff, UK.
27
Centre for Cancer Research and Cell Biology, Queen's University Belfast, Belfast, UK.
28
Department of Clinical Oncology, East Lancashire Hospitals NHS Trust, Blackburn, UK.
29
Northern Centre for Cancer Care, Freeman Hospital, Newcastle upon Tyne, UK.
30
Clinical Oncology, Singleton Hospital, Swansea, UK.
31
Exeter Oncology Centre, Royal Devon & Exeter Hospital, Exeter, UK.
32
Department of Oncology, Shrewsbury and Telford Hospitals NHS Trust, Shrewsbury, UK.
33
Department of Clinical Oncology, The Christie NHS Foundation Trust, Manchester, UK.
34
Medical Research Council (MRC) Clinical Trials Unit at University College London (UCL), London, UK. Electronic address: mrcctu.stampede-publications@ucl.ac.uk.
- 덧글달기










편집위원
전이성 전립선암에서 원발병변에 대한 방사선치료의 이득을 증명한 3상 임상연구로, 환자 선택과 연구디자인의 중요성을 보여줌.
2019-01-23 14:00:14
편집위원
전이성 전립선암 환자에서 원발부위에 대한 방사선치료가 생존율을 증가시키지 못했지만, subgroup analysis를 시행하였을 때, low metastatic burden을 동반한 환자들에서는 유의한 향상이 있음을 보여준 연구로, 전이성 전립선암 환자에서 국소 치료로서 방사선치료를 적용할지 결정하는데 도움이 될 수 있는 좋은 연구입니다.
2019-01-23 14:02:55