글로벌 연구동향
핵의학
- [EJNMMI Res.] 동맥혈전증 PET 1상임상시험A phase 1, first-in-human study of 18F-GP1 positron emission tomography for imaging acute arterial thrombosis.
울산의대 / 채선영, 권태원, 진소영, 문대혁*
- 출처
- EJNMMI Res.
- 등재일
- 2019 Jan 7
- 저널이슈번호
- 9(1):3. doi: 10.1186/s13550-018-0471-8.
- 내용
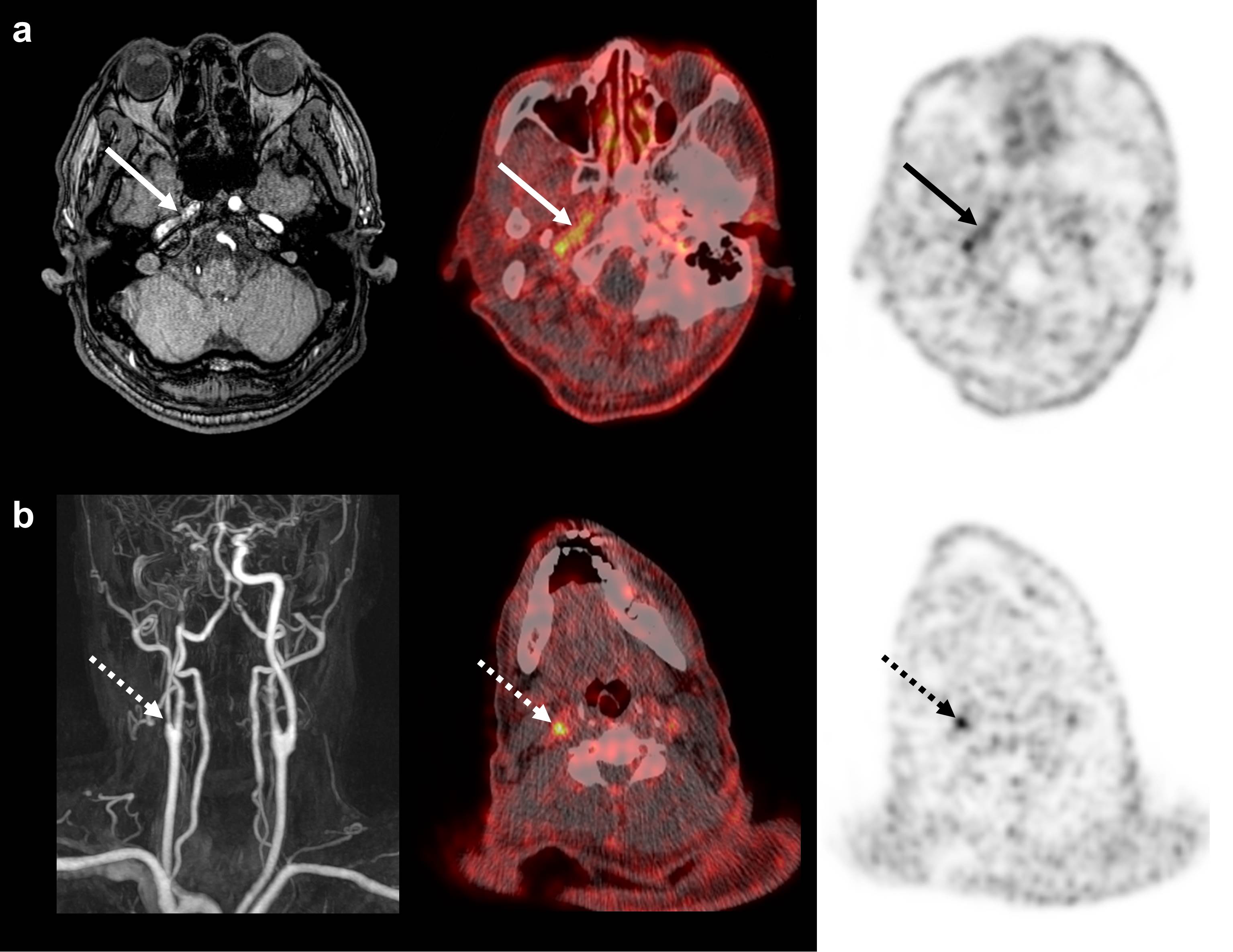
오른쪽 중뇌동맥 영역과 기저핵 부위에 급성 뇌경색을 보인 61세 남자환자의 18F-GP1 PET/CT 영상. 자기 공명 영상에서 충만 결손이 있는 우측 내경동맥 추체부와 (a, 화살표) 우측 근위 내경동맥에 (b, 점선 화살표) 국소적인 18F-GP1 섭취 증가가 있음.
Abstract
BACKGROUND:
18F-GP1 is a novel positron emission tomography (PET) tracer that targets glycoprotein IIb/IIIa receptors on activated platelets. The study objective was to explore the feasibility of directly imaging acute arterial thrombosis (AAT) with 18F-GP1 PET/computed tomography (PET/CT) and to quantitatively assess 18F-GP1 uptake. Safety, biodistribution, pharmacokinetics and metabolism were also evaluated.METHODS:
Adult patients who had signs or symptoms of AAT or had recently undergone arterial intervention or surgery within 14 days prior to 18F-GP1 PET/CT were eligible for inclusion. The AAT focus was demonstrated by conventional imaging within the 5 days prior to 18F-GP1 administration. Whole-body dynamic 18F-GP1 PET/CT images were acquired for up to 140 min after injection of 250 MBq of 18F-GP1. Venous plasma samples were analysed to determine 18F-GP1 clearance and metabolite formation.RESULTS:
Among the ten eligible patients assessed, underlying diseases were abdominal aortic aneurysm with endovascular repair (n = 6), bypass surgery and stent placement (n = 1), endarterectomy (n = 1), arterial dissection (n = 1) and acute cerebral infarction (n = 1). 18F-GP1 administration and PET/CT procedures were well tolerated, with no drug-related adverse events. All patients showed high initial 18F-GP1 uptake in the spleen, kidney and blood pool, followed by rapid clearance. Unmetabolised plasma 18F-GP1 levels peaked at 4 min post-injection and decreased over time until 120 min. The overall image quality was sufficient for diagnosis in all patients and AAT foci were detected in all participants. The 18F-GP1 uptake in AAT foci remained constant from 7 min after injection and began to separate from the blood pool after 20 min. The median standardised uptake value of AAT was 5.0 (range 2.4-7.9) at 120 min post-injection. The median ratio of standardised uptake value of AAT foci to the mean blood pool activity was 3.4 (range 2.0-6.3) at 120 min.CONCLUSIONS:
18F-GP1 is a safe and promising novel PET tracer for imaging AAT with a favourable biodistribution and pharmacokinetic profile.TRIAL REGISTRATION:
ClinicalTrials.gov identifier: NCT02864810 , Registered August 3, 2016.
Author informationChae SY1, Kwon TW2, Jin S3, Kwon SU4, Sung C1, Oh SJ1, Lee SJ1, Oh JS1, Han Y2, Cho YP2, Lee N5, Kim JY6, Koglin N7, Berndt M7, Stephens AW7, Moon DH8.
1
Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Republic of Korea.
2
Department of Vascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
3
Department of Nuclear Medicine, Nowon Eulji Medical Center, Eulji University, Seoul, Republic of Korea.
4
Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
5
Department of Nuclear Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Republic of Korea.
6
Department of Nuclear Medicine, Guri Hospital of Hanyang University Medical Center, Hanyang University College of Medicine, Seoul, Republic of Korea.
7
Life Molecular Imaging GmbH (formerly Piramal Imaging GmbH), Berlin, Germany.
8
Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Republic of Korea. dhmoon@amc.seoul.kr.
- 키워드
- 18F-GP1; Arterial thrombosis; Glycoprotein IIb/IIIa receptor; Platelet activation; Positron emission tomography
- 연구소개
- 18F-GP1은 활성화된 혈소판의 glycoprotein IIb/IIIa 수용체를 표적으로 하며, 급성기의 활동성 혈전을 영상화 할 수 있는 새로운 진단 기법입니다. 국내외 첫 인체를 대상으로 한 이번 임상시험을 통해, 18F-GP1이 급성 동맥 혈전증을 영상화 하는데 있어서 적합한 생체분포와 약동학을 보이는 안전하고 유망한 PET 용 방사성의약품임을 확인 할 수 있었습니다. 향후 다양한 혈전성 혈관질환의 진단 및 치료방향 결정에 있어서 18F-GP1 PET/CT의 임상적 활용 가능성이 높을 것으로 기대됩니다.
- 덧글달기










편집위원
Arterial thrombus formation & relative event가 생길 위험도가 높은 병변을 찾아 내는 것은 임상적으로 매우 중요하지만 아직까지 쉽지 않은 문제임. 이런 상황에서 promising한 novel PET tracer를 개발하고 임상시험 단계에 진입했다는 것은 의미 있는 일이며 보고된 biodistribution & pharmacokinetic 결과도 매우 고무적으로 독자들에게 소개할 만한 가치가 있다고 생각함.
2019-02-22 17:18:34