글로벌 연구동향
핵의학
- [Int J Mol Sci.] Regulated Mesenchymal Stem Cells Mediated Colon Cancer Therapy Assessed by Reporter Gene Based Optical Imaging.
경북의대 / Senthilkumar Kalimuthu, 안병철*
- 출처
- Int J Mol Sci.
- 등재일
- 2018 Mar 27
- 저널이슈번호
- 19(4).
- 내용
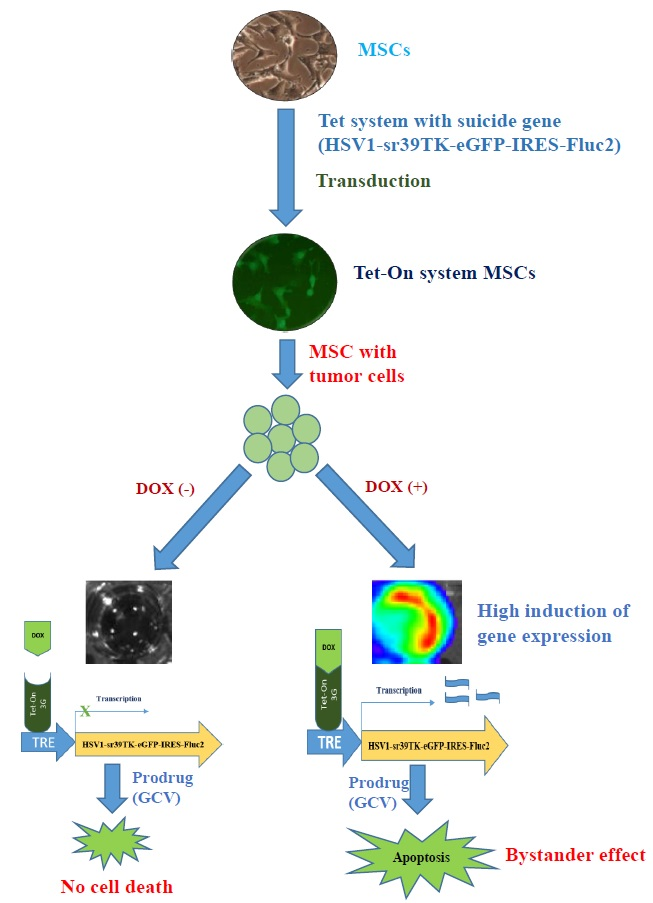
Abstract
Colorectal cancer is the most common cancer in both men and women and the second most common cause of cancer-related deaths. Suicide gene-based therapy with suicide gene-transduced mesenchymal stem cells (MSCs) is a promising therapeutic strategy. A tetracycline-controlled Tet-On inducible system used to regulate gene expression may be a useful tool for gene-based therapies. The aim of this study was to develop therapeutic MSCs with a suicide gene that is induced by an artificial stimulus, to validate therapeutic geneexpression, and to monitor the MSC therapy for colon cancer using optical molecular imaging. For our study, we designed the Tet-On system using a retroviral vector and developed a response plasmid RetroX-TRE (tetracycline response element) expressing a mutant form of herpes simplex virus thymidine kinase (HSV1-sr39TK) with dual reporters (eGFP-Fluc2). Bone marrow-derived MSCs were transduced using a RetroX-Tet3G (Clontech, CA, USA) regulatory plasmid and RetroX-TRE-HSV1-sr39TK-eGFP-IRES-Fluc2, for a system with a Tet-On (MSC-Tet-TK/Fluc2 or MSC-Tet-TK) or without a Tet-On (MSC-TK/Fluc2 or MSC-TK) function. Suicide gene engineered MSCs were co-cultured with colon cancer cells (CT26/Rluc) in the presence of the prodrug ganciclovir (GCV) after stimulation with or without doxycycline (DOX). Treatment efficiency was monitored by assessing Rluc (CT26/Rluc) and Fluc (MSC-Tet-TK and MSC-TK) activity using optical imaging. The bystander effect of therapeutic MSCs was confirmed in CT26/Rluc cells after GCV treatment. Rluc activity in CT26/Rluc cells decreased significantly with GCV treatment of DOX(+) cells (p < 0.05 and 0.01) whereas no significant changes were observed in DOX(-) cells. In addition, Fluc activity in also decreased significantly with DOX(+) MSC-Tet-TK cells, but no signal was observed in DOX(-) cells. In addition, an MSC-TK bystander effect was also confirmed. We assessed therapy with this system in a colon cancer xenograft model (CT26/Rluc). We successfully transduced cells and developed a Tet-On system with the suicide gene HSV1-sr39TK. Our results confirmed the therapeutic efficiency of a suicide gene with the Tet-On system for colon cancer. In addition, our results provide an innovative therapeutic approach using the Tet-On system to eradicate tumors by administration of MSC-Tet-TK cells with DOX and GCV.
Author information
Kalimuthu S1, Zhu L2, Oh JM3, Lee HW4, Gangadaran P5, Rajendran RL6, Baek SH7, Jeon YH8, Jeong SY9, Lee SW10, Lee J11, Ahn BC12.
Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. abc2000@knu.ac.kr.
1 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. senthilbhus@gmail.com.
2 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. liyazhu1001@gmail.com.
3 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. ojm0366@naver.com.
4 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. howonlee1234@gmail.com.
5 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. prakashg@knu.ac.kr.
6 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. ramyasep9@gmail.com.
7 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. bsh4498@naver.com.
8 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. jeon9014@gmail.com.
9 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. syjeongnm@gmail.com.
10 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. swleenm@knu.ac.kr.
11 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. jaetae@knu.ac.kr.
12 Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu 41944, Korea. abc2000@knu.ac.kr.
- 키워드
- colon cancer; herpes simplex virus thymidine kinase (HSV1-sr39TK); mesenchymal stem cells (MSCs); optical imaging; suicide gene therapy
- 연구소개
- 간엽줄기세포에 tetracycline inducible promoter 체계에 의해 발현이 조절되는 thymidine kinase를 이입하고, 이를 Ganciclovir 와 함께 투여하여 대장암 치료에 이용한 세포 치료술 개발에 대한 연구입니다. 종양치료술의 효과는 광학분자영상기술로 주로 평하였습니다. 해당 논문은 세포치료술에 관심이 있는 기초연구자 및 분자영상을 이용한 동물실험에 관심이 있는 연구자들에게 도움이 될 것이라 생각합니다.
- 덧글달기









